Introduction

Carbon is one the most-important elements in agricultural soils. The following article takes an in-depth look at how carbon is understood, measured, and managed to benefit soil health in agricultural production.
Article Topics
- Soil Carbon Cycle
- Organic Carbon Cycle
- Inorganic Carbon Cycle
- Carbon Measurements
- Collection of Samples to Measure Soil Carbon
- Soil Carbon Stocks and Carbon Credits
- Management to Improve Carbon Indicators of Soil Health
- Summary and References
Soil Carbon Cycle
Carbon is one the most-important elements in agricultural soils. Carbon in the soil is found in forms created by life (organic) and by mineralization and dissolution of chemicals (inorganic). Carbon in soils is important for the formation of soil structure, nutrient retention and cycling, and agricultural production. Soils with a greater organic carbon concentration generally have better agricultural productivity. Increasing soil organic carbon content can improve soil structure, nutrient retention and microbial activity in the soil. Policymakers and many others are interested in the soil’s role in the environmental carbon cycle.
The element carbon is contained in the atmosphere, biosphere and geosphere. The atmosphere is the gas containing layer of earth. The biosphere is the layer of earth where organisms are living, including near-surface geologic materials and organisms living on the surface of the earth. The geosphere is the layers of minerals and rocks below the biosphere. Carbon found in the atmosphere is primarily in a gaseous form such as carbon dioxide (abbreviated as CO2) and methane (abbreviated as CH4). The carbon in the biosphere consists of carbon found in soil, fresh and salt waters, and living organisms within or on the surface of the biosphere. The lithosphere contains carbon that is stored in minerals, such as limestone, coal and oil. Carbon can be moved from one pool to another by organisms or chemical reactions. An example of the global carbon cycle is shown in Figure 1. Soils are at the interface between the atmosphere and lithosphere and play a large component in the biochemical carbon cycle.
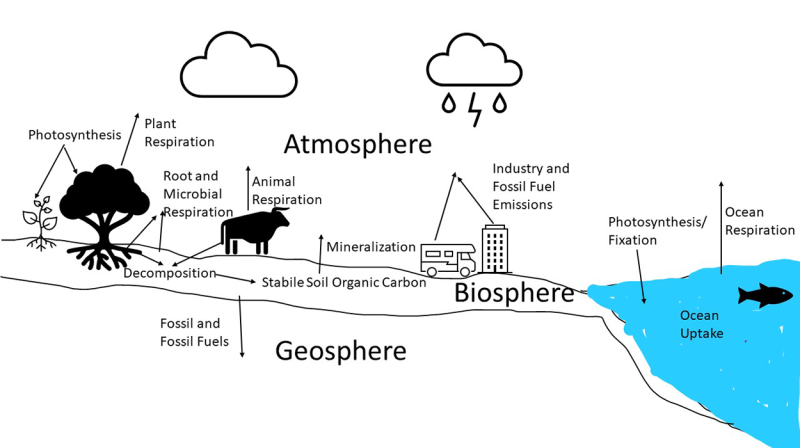
Carbon is a very important component of the soil matrix. Carbon in the soil can be living, dead or in inorganic forms in the soil. Living sources of carbon include growing plants, fungi, bacteria and other organisms that reside in the soil. Dead organic carbon sources include dead plants and other organisms and organic compounds that are contained in the soil. Organic compounds found within the soil include sugars, carbohydrates, proteins, lignin, cellulose, and fulvic and humic acids. The soil organic carbon pool is made up of three pools, which are the active pool, slow pool and recalcitrant pool. Pools are different fractions or reservoirs of carbon compounds that are contained within the biospheres or other earth layers. The active carbon pool is soil organisms and labile soil carbon, such as dead plant matter which may be broken down in a short time (months to a few years). The slow pool is organic carbon that will be broken down in over a decade’s time. Recalcitrant carbon is carbon that will remain in the soil for a long period of time and is comprised of complex carbon compounds, such as humic substances. Inorganic carbon is carbon that is precipitated in chemicals, such as calcium carbonate, or is part of the mineral parent material of the soil. The most-common soil parent material that contains carbon is limestone or dolomite. However, through biochemical processes, precipitated inorganic carbon can be formed in the soil.
Organic Carbon Cycle
Photosynthesis occurs from plants taking carbon dioxide from the atmosphere and turning it into an organic form of carbon. An example of the soil carbon cycle is in Figure 2. Plants absorb light and use chlorophyll to transform carbon dioxide into carbohydrates, then release oxygen back into the air. Plants store the carbon that they fix in both above-ground and below-ground biomass. Both above and below ground carbon that is fixed by the plant can be involved in the microbial decomposition process in the soil. However, some bacteria, such as cyanobacteria, also are able to fix carbon from the atmosphere and convert CO2 into sugars.

Plants also need to respire to remove excess CO2 gas through a process called respiration. Plants take in oxygen and use sugars in the plant for energy. When processing sugars for energy, CO2 is produced. This occurs in both the above-ground and below-ground portion of the plant. Macro and microorganisms living above and below the soil surface also respire, which releases CO2 into the atmosphere. Additionally, livestock operations also release carbon into the atmosphere as CO2 from livestock that are respiring and the decomposition of the manures.
Mineralization /decomposition is the breakdown of organic carbon into CO2 in the soil. Types of carbon that can be mineralized include above and below ground plant materials, fungi, bacteria, above and below ground biota, and stabilized organic carbon in the soil. Soil macro and microbiota eat plants or other carbon sources present in the soil and break it down into smaller organic carbon compounds to use carbon as a source of energy. Types of carbon that are easily used by microorganisms for food and mineralized in the soil include sugars, carbohydrates, lipids, and amino acids. Some of the carbon sources that are used by microbes are broken down and converted to CO2, whereas others are converted into complex carbon compounds that can be transformed into the slow or recalcitrant carbon pool. During mineralization, organic carbon sources are converted to CO2, and the gas is released into the atmosphere. However, some of the carbon compounds formed by soil biota can also be stabilized to sources of carbon that remain in soil for a long period of time in the soil (recalcitrant pool). Carbon sources that are stabilized soil organic carbon can be mineralized into CO2 also and the CO2 gas returns back to the atmosphere. Mineralization also occurs from decomposition of manures from livestock, which can return carbon in gaseous forms to atmosphere.
Stabilization of organic carbon present in the soil into compounds that will remain in the soil for long durations of time is what many people are interested in. This is due to the ability of the soil to remove carbon in the gaseous form from the atmosphere and convert it to a form that is stable for a long period of time in the soil. Some carbon sources that form during the microbial decomposition of carbon compounds in the soil are complex compounds, such as fulvic and humic compounds, which can remain in the soil for a long period of time. Carbon can be stabilized physically, chemically, and biologically in the soil. Physical stabilization is storage of carbon containing compounds into soil aggregates. The sources of carbon that can be stabilized into soil aggregates include chemical compounds formed from the mineralization of carbon and particulate organic matter from above and below ground organism biomass. Organic carbon also can be chemically stabilized by being absorbed to chemical compounds in the soil, such as clays. This process generally occurs with more complex carbon compounds that are formed through the mineralization of organic carbon sources. Soils with higher clay content generally can contain a higher quantity of soil organic carbon that is chemically stabilized. Soil biology processes fresh carbon sources in the soil. The carbon sources formed during microbial decomposition are then transformed into compounds that can diffuse into soil aggregates and be stabilized (Kravchenko et al., 2019). Climate, carbon sources present, and the soil microbial community present affect the rate that carbon is processed in the soil (Bhattacharyya et al., 2022). Stabilized carbon sources present in the soil can be mineralized/decomposed by soil microorganisms also. This will cause stabilized (recalcitrant pool) organic carbon to be converted to CO2. Agricultural producers and others interested in carbon sequestration in soils want to increase carbon that is stabilized in the soil and reduce the amount of carbon released to the atmosphere as CO2 by mineralization.
In anaerobic conditions, certain microorganisms use inorganic chemicals as an electron source instead of oxygen. The chemicals that are used by microorganisms depend upon the oxidation-reduction potential of the soil. When soil is not saturated, the redox potential is high, and oxygen is used as the electron source. As the redox potential decreases, different chemical compounds are used as an electron source. The order of chemicals being used with decreasing redox potential in the soil are Oxygen (abbreviated as O2)> Manganese (abbreviated as Mn4+)> iron (abbreviated as Fe3+) > Sulfate (S6+) > carbon dioxide (C4+). During this process, these chemicals are transformed from a more-oxidized form (>positive electron charge) to a more-reduced form (more-negative electron charge). For example, during the formation of methane (methanogenesis), carbon is transformed from an electron charge of +4 (abbreviated as C4+) to an electron charge or -4 (abbreviated as C4-). There are 8 electrons released during this process, which are used by microorganisms to break down carbon and water, and methane (abbreviated as CH4) is created. Methanogenesis is a cycle that occurs during anaerobic conditions. The microorganisms that participate in the methane formation are archea. This process most commonly occurs in areas that are saturated with water, and oxygen is not present for microbes to use. This commonly occurs in wetlands and after rainstorms in fully saturated soils. Organisms in soils also can use methane as an energy source when it moves to a soil environment where there is oxygen present. Organisms also can release methane into the atmosphere during digestion. Ruminant animals release methane during digestion, which is commonly referred to as enteric methane emissions. Some people are concerned about methane being released from the soil and agriculture and think that it is a worse gas to have in the environment than carbon dioxide.
Inorganic Carbon Cycle
Inorganic carbon is also contained in soil and is formed by chemical reactions and not by living organisms. Inorganic carbon is more common in semi-arid and arid soils and is most commonly in the form of calcite. However, producers commonly add inorganic carbon to soils in the form of lime to increase the soil pH, which is mostly calcium carbonate. Bicarbonate (abbreviated as HCO3-) and hydrogen in the form of H+ are formed in the soil from carbon dioxide and water. The amount of hydrogen and carbon dioxide formed depends upon the concentration of carbon dioxide present in the atmosphere/soil (Guo et al., 2016). The equations for the chemical reaction that forms carbonic acid in the water/soil and the precipitation of calcite are outlined in Equations 1 and 2.
Equation 1:
CO2 + H2O ↔ HCO3- + H+
Equation 2:
Ca2+ + 2HCO3- ↔ CaCO3 + H2O + CO2
Then calcium and bicarbonate can react to form calcium carbonate, water, and carbon dioxide. The amount of calcite produced in the soil depends upon the soil pH. At a higher soil pH (>7.8) there is precipitation of calcite occurring in the soil. When the soil pH is acidic, calcium carbonate in the soil can dissolve and become water, carbon dioxide, and dissolved calcium. As concentration of carbon dioxide present in the air increases, water/soil will become more acidic (lower pH) and more of the calcite in the soil will dissolve. Reducing the soil pH can reduce the soil inorganic carbon concentration. However, for agricultural production, maintaining a pH around 7 is ideal.
Carbon Measurements
Total Carbon
Total carbon is the total amount of carbon in the soil. It contains both organic and inorganic carbon that is present in the soil. Total carbon is usually measured by wet or dry combustion method (Nelson and Sommers 1996). Dry combustion is the most-used technique to measure total carbon in the soil. The soil is placed into an instrument that is at a high temperature >1832 degrees Fahrenheit (abbreviated as °F), then carbon dioxide released from sample is measured, which is converted into carbon concentration in the soil sample. Wet combustion uses potassium dichromate and sulfuric acid to oxidize the carbon in the soil and then measures potassium dichromate concentration remaining in solution using a spectrophotometer.
Inorganic Carbon
Inorganic carbon is most commonly found in the soil as carbonates. Carbonates found in the soil are primarily calcium carbonate, but also could be magnesium carbonates. Carbonates are most commonly found in the subsoil horizons. Carbonates are not a living source of carbon in the soil but can be dissolved when soil solution is acidic (pH < 7). Soils or layers of soil that are high in carbonates are frequently white colored. A quick way to see if the soil has carbonates is to pour an acid, such as vinegar, on the soil and see if it fizzes. Inorganic carbon in soil is frequently measured by a calcimeter or by titration with an acid (Sherrod et al., 2002 and Bundy and Bremner 1972). When using the titration technique, an indicator that shows when soil pH changes from a base to an acid is added to the soil and solution. Acid is added to the sample until the indicator changes color and the amount of acid added is equal to inorganic carbon concentration of the soil. The pressure calcimeter technique measures pressure of gas that is released from the soil when the soil and an acid are mixed together and placed in a closed container. The greater the inorganic carbon concentration is in the soil, the greater the pressure will be in the calcimeter from the production of CO2 gas.
Organic Carbon
Organic carbon consists of living microorganisms in the soil, material from plants, and organic compounds that have been formed through the decomposition of organic materials. Organic carbon is the type of carbon that is more important to soil health than inorganic carbon. Many agricultural soils do not have a high pH (>7.8), so total carbon is considered to be equal to organic carbon concentration. However, in semiarid areas, like South Dakota, soil pH is high and inorganic carbonates are present. Inorganic carbon concentration needs to be accounted for when measuring soil organic carbon concentration and determining organic carbon stocks. One way to measure soil organic carbon is to do a pretreatment with an acid, such as sulfuric acid. Then the sample is placed into a dry combustion total carbon analyzer. The Walkey and Black method uses potassium dichromate and sulfuric acid to oxidize the organic carbon in the soil (Nelson and Sommers 1996). Then the potassium dichromate remaining in solution after oxidation is determined. Organic carbon also can be determined by subtracting measured inorganic carbon from the total carbon concentration.
Soil Organic Matter
Soil organic matter measures carbon contained in the soil, plus the other elements contained in the carbon compounds. Soil organic matter is approximately 58% carbon. Many soil laboratories measure soil organic matter as a routine soil analysis. Soil organic matter is most commonly measured by loss on ignition at a temperature of around 842 °F. Soil is dried in a oven to remove water at 220 °F, weighed, then placed in a furnace. Weight lost in the furnace is divided by total soil mass to determine percent soil organic matter. However, at deeper depths in soils which contain carbonates, carbonates may be lost during combustion, leading to inaccuracies in this measurement when soils have high carbonate (inorganic carbon) concentrations.
Soil Microbial Biomass
Soil microbial biomass carbon is a fraction of soil carbon that consists of microbes, such as bacteria, fungi, and protozoa. Microbiology is essential for soil biochemical processes to occur. Soil microbial biomass carbon is frequently about 2 to 5% of the total soil organic carbon (Vance et al., 1987). Soil microbial biomass carbon is most commonly measured using the fumigation extraction method. A fumigation incubation method also is used to measure soil microbial biomass. To extract the microbial biomass the soil is treated with choroform to kill the microbes. When using the incubation method, the chloroform-treated sample is incubated, and carbon dioxide released from the sample is measured (Joergensen et al., 2011). When using the extraction method, dead microorganisms in the soil are extracted, and carbon content of extracted cells is determined using potassium dichromate solution and back titration (Joergensen et al., 2011).
Permanganate-Oxidizable Carbon (POXC)
POXC is permanganate oxidizable carbon. It is a measurement of carbon types that are considered to be active in the soil. The carbon forms that are considered to be active include particulate organic matter from plant roots or above-ground plants, carbohydrates, sugars, and amino acids. Active or labile carbon is more rapidly affected by management practices than total organic carbon. POXC is determined by placing air dry soil into a potassium permanganate solution (abbreviated as KMn04) (Weil et al., 2003). The permanganate in the solution then oxidizes these carbon compounds. After the carbon is oxidized, the color of the permanganate solution-soil mix is measured with a spectrophotometer to determine light absorbance. The light absorbance in the liquid then can be correlated to how much carbon was oxidized in the soil sample.
Mineralizable Carbon
Mineralizable carbon or CO2 burst is a measurement that can determine the amount of carbon that is processed and mineralized by soil microbes when the soil is wet to a certain moisture content. To conduct this procedure, the soil is placed into an enclosed glass jar. The soil is wet to a moisture content, such as 50% water content. Then the soil is incubated for a period of time and the carbon dioxide released from the sample is measured (Wade et al., 2018). This incubation is usually done for 1 to 3 days. The amount of carbon dioxide produced during the incubation period is dependent upon easily processed carbon (labile) and microbial biomass present and the potential of the soil to mineralize nutrients (Hurriso et al., 2016). Soils with higher carbon mineralization rate generally have more microbes that are active and can mineralize nutrients that are essential to plants more rapidly.
Water-Extractable Organic Carbon
Water-extractable organic carbon is carbon in the soil that is dissolved in water. The carbon that dissolves in water is humic acids, sugars, carbohydrates, and amino acids. These carbon compounds are formed through the decomposition of litter and humus in soil (Kalbitz et al. 2001). Soils with low amounts of water-extractable carbon likely have low amounts of litter being returned to them and biological activity. Water-extractable carbon can be measured with both cold water (68 °F) or hot water (176 °F). Cold-water extractable carbon contains more-easily soluble carbon compounds, such as carbonates and sugar in soil, whereas hot water can extract more humic and protein-like compounds present in the soil (Zhao et al., 2013 & Landgraf et al., 2006). Hot-water extractable carbon is usually higher than cold-water extractable carbon due to more mineralization of carbon in warmer water and increased solubility of certain carbon-containing compounds (Ćirić et al., 2016; Landgraph et al., 2006). Soils are placed into deionized water and mixed, then water is extracted from the sample. Carbon that is contained in the sample is then measured by absorbance using a spectrophotometer (Zsolnay 1996). Dissolved organic carbon also can be measured using a total carbon analyzer, but the inorganic carbon concentration in the soil water needs to be corrected for (Ghani et al., 2003).
Acid-Extractable Carbon
Acid-extractable carbon, or acid hydrolysis, is another method used to determine labile carbon in the soil. Soil is placed into a 1 or 6 molar hydrochloric acid solution, then some of the organic carbon in soil is oxidized and CO2 is released from the sample. CO2 released from the sample is then measured, and non-hydrolysable carbon is determined by subtracting carbon in CO2 released from total carbon (Paul et al., 2006). The acid dissolves inorganic carbon and oxidizes carbon present is in the form of fatty acids, proteins and polysaccharide,s but leaves more-stable carbon sources in the soil, such as alkyls, lignin, and other aromatic carbon sources in the soil (Paul et al., 2006). The non-hydrolysable carbon present in the soil is carbon that would fall into the recalcitrant carbon pool, which can become stabilized in the soil.
Particulate Organic Matter
Particulate organic matter is organic matter that is in the soil considered to be larger than silt sized (53 um or 0.0021 in.). Most of the particulate organic matter present in the soil is from plant roots, leftover plant materials, and microorganisms present in the soil. Particulate organic matter is determined by dispersing the soil with sodium hexametaphosphate, shaking the soil on a shaker, then the soil is wet-sieved through a 53 um (0.0021 in.) sieve. Other size sieves can be nested on top of the 53um sieve to separate the particulate organic matter into coarse (> 500 um or 0.02 in.) and fine (0.0021 to 0.02 in.) or other size fractions. One method for determining particulate organic matter is loss on ignition of the air-dry mass retained of the sieve (Cambardella et al., 2001). This gives you the organic matter content of the particulate organic matter contained in a soil, which includes carbon plus other elements in organic compounds. Researchers also have measured particulate organic matter carbon content by dry combustion using a total carbon analyzer (Witzgall et al., 2021).
Soil Gas Emissions

Soil gas emissions can be measured by sensors or by collecting gas emitted into a chamber over a duration of time. Sensors can be purchased that can measure specific gases emitted from soil, such as CH4 and CO2. Static chambers also are used, which can determine how much gas is released in a period of time in the area of the chamber. Some of these chambers can measure certain gases that are released into the chamber, such as the Licor in Figure 3. Samples of a certain volume also can be taken from the chamber throughout a period of time to determine how much of a certain gas is released during a period of time. The samples are then run on a gas chromatograph. An example of a chamber used to measure soil gas emissions is shown in Figure 3.
Carbon Compounds
Some researchers also are measuring certain types of carbon compounds that are contained in soils. Some of the carbon compounds that can be measured include sugars, carbohydrates, fulvic and humic acids, amino acids, bacteria, and fungi. Different extraction techniques can be used to separate these compounds from the soil. These organisms are then identified by techniques used to identify organic chemicals or markers of certain organisms. One way that is used to identify certain organisms in the soil is fatty acid methyl ester profiles (Cavigelli et al., 1995). Another way to identify certain soil organisms is by soil DNA extraction.
Collection of Samples to Measure Soil Carbon
Much of the organic carbon that is contained in the soil is near the soil surface. However more inorganic carbon is deeper in the soil profile. Soil carbon related properties also can vary spatially throughout the field. If measuring soil carbon stocks, you may want to sample to a deeper depth to find carbon concentration that is stored in the entire soil profile. Samples to analyze properties indicative of labile or more-active carbon sources are mostly done near the surface. Soil organic carbon is higher near the soil surface. In the darker area in the soil pit pictured in Figure 4, there is more organic carbon, whereas deeper in the soil profile where there are white spots, there is more inorganic carbon. The white appearing spots are calcium carbonate. Most of the labile carbon, such as the straw on the surface and near surface roots, is where most of the carbon that is easily used by microbes is located.

Changes in agricultural management practices will initially cause soil properties to change near the soil surface, so if sampling to monitor changes in soil carbon properties from management, samples should be taken near surface, such as from the 0 to 6 and 6 to 12-inch depths. If sampling to determine the soil total and organic carbon stocks, samples should be taken to a deeper depth, such as 0 to 36 inches. Sampling can be done by collecting a point sample, a composite sample, or as grid sampling. If collecting a composite sample, multiple samples should be collected throughout the field of interest to a desired depth. The samples collected from a given depth range then should be homogenized together prior to the analysis of soil carbon indicators. If grid sampling, the field is set up into a grid, and samples are collected at certain points in the field. Collecting a composite sample and sending it to the lab is cheaper, but it will not account for variation in soil carbon throughout the field. Collecting a point sample may not be able to show how soil properties are changing throughout the entire field. If determining soil carbon stock, bulk density of the soil also should be collected. When collecting samples for soil carbon analysis, they should be kept cool and sent to the desired lab as soon as possible to be processed. If they cannot be sent to a lab to be processed as soon as possible they, should be air-dried prior to sending to the lab.
Soil Carbon Stocks and Carbon Credits
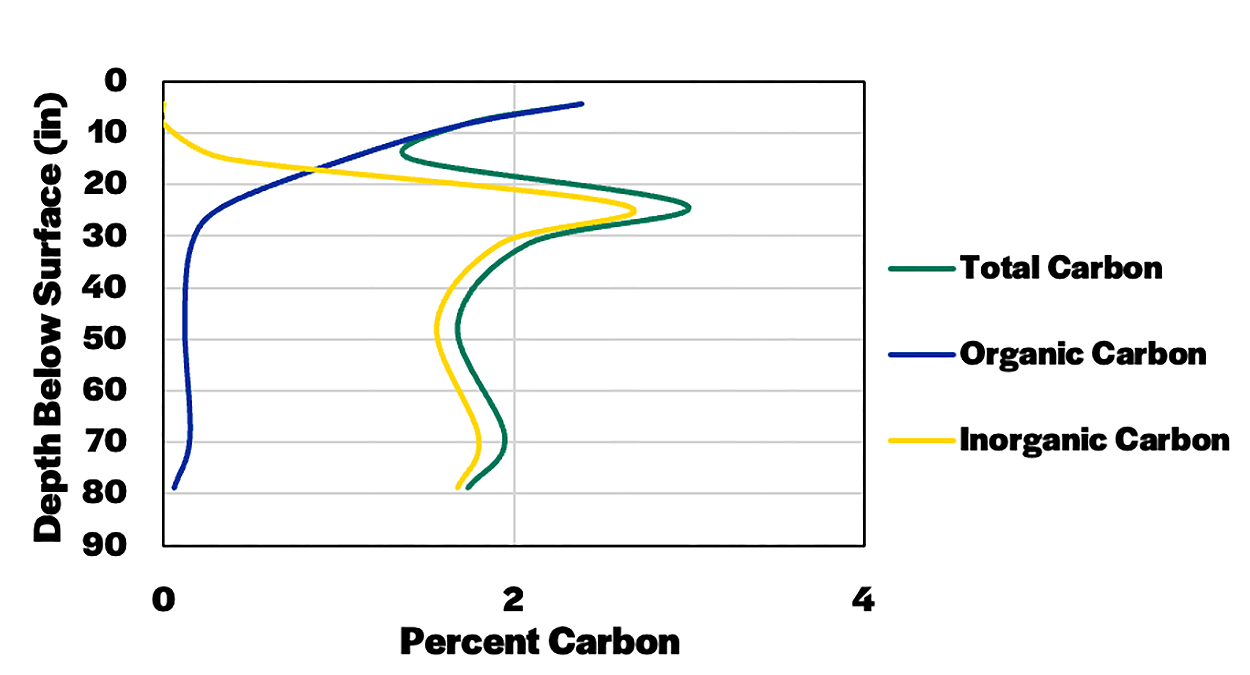
Policymakers and companies are interested in the soil's ability to store organic carbon and remove carbon from the atmosphere that is in the form of CO2 and CH4. Soil carbon stock is the tons of carbon per-acre stored in a certain soil depth. Carbon stock can be calculated by multiplying the percentage of carbon × bulk density × soil depth × area in an acre. For example, a soil that is 1.96 % carbon in the 0-2.5 in. (0-0.164 ft.) depth and has a bulk density of 60.4 lbs. ft-3 has 0.19 lbs. carbon ft-2, or 8464 lbs. (4.23 tons) of total carbon per acre in the 0-2.5 in depth. An example of soil total, organic and inorganic carbon concentration versus depth and stocks of carbon at the depth ranges sampled is found in Figure 5 and Figure 6 below. This data is of a Houdek soil, which has 283 tons of total carbon, 57 tons of organic carbon, and 226 tons of inorganic carbon per-acre in the 0-78 in. depth range (Figure 6). There is more organic carbon near the soil surface, whereas inorganic carbon is highest in the 23.5-31.5 in. depth (Figure 5). Organic carbon concentration is usually higher near the soil surface, whereas inorganic carbon values vary depending upon soil parent materials and climate. In older soils and where annual precipitation is higher, there generally is little inorganic carbon near the soil surface, except when lime (CaCO3) is added near the soil surface. In arid or semi-arid areas, carbonates generally are not leached out of the soil profile in the top 6 ft.

Soil is considered to be an important sink of carbon, and people are interested in the soil's ability to grow plants, which can convert carbon as CO2 in the atmosphere to organic carbon forms. CO2 and CH4 are greenhouse gases emitted through the carbon cycle that may affect weather. Carbon credit is the price of what a ton of CO2 costs. Companies that emit CO2 will pay for credits to offset the carbon that they have emitted to say they are carbon neutral. Agricultural producers will then get paid to conduct practices that can remove carbon from the atmosphere and convert it to organic forms. Practices that brokers of credits will pay agricultural producers to do is reduced or no tillage, growing cover crops, retaining crop residues, improving grazing practices, planting trees, converting annual cropland to perennial vegetation, and increasing density of trees on the landscape (Sellers et al., 2021). These practices, in theory, can remove CO2 from the atmosphere via carbon fixation and convert carbon in the form of CO2 in the atmosphere to organic carbon in plant biomass both above and below ground. Some of the carbon in the additional biomass produced from new management practices, such as planting cover crops, trees or rotational grazing, then is converted to a stabilized (recalcitrant) carbon pool in the soil. These companies also consider carbon in tree or other plant biomass to be removal of carbon from atmosphere and to count towards credits.
If a producer decides to implement a practice that a carbon broker company will pay them to do, a cost/benefit analysis should be conducted. For example, if what the carbon credit company is paying you annually to plant cover crops is less than what seed and planting costs are for planting cover crops, it may not be beneficial to the producer to enroll in the carbon credit practice. Some companies also will test soil for carbon prior to initiation of the practice, then test the soil at a later date to see if the implemented practice is actually increasing soil carbon stocks.
Management to Improve Carbon Indicators of Soil Health

Carbon is the most-essential element in the soil for maintaining soil health. As previously mentioned, some management practices that can increase soil organic carbon are planting cover crops, reducing tillage intensity, planting perennial vegetation, and retaining crop residues (Lal 2013). Also, applying organic materials, such as animal manures or compost, to the soil can increase soil organic carbon and active carbon in the soil (Li et al.2018). In relatively short durations of time, these management practices generally only increase soil organic carbon near the surface. Many of the soil carbon indicators measured in soil health are indicators of active carbon, which are affected by amount of carbon being annually returned to the soil and microbial activity occurring in the soil. To improve the soil properties that are indicators of active or labile carbon, producers should use management practices that return more carbon to the system, such as cover crops, animal manure application, diversifying the cropping system, leaving straw or stover in field, or using practices that minimize disturbance to soil aggregates, which slows the mineralization rate of carbon in the system. The carbon-to-nitrogen ratio of the soil and materials being applied to the soil may affect the percent makeup of certain carbon compounds in the total soil carbon concentration. Management practices that may lead to a reduction in soil pH, such as applying nitrogen fertilizers or chemicals that reduce soil pH, may lead to a reduction in soil inorganic stock. However, irrigation with water high in carbonate and bicarbonate can increase soil inorganic carbon stock.
Summary
Carbon is an essential element to life and is the most important chemical to soil health. Carbon is contained in the atmosphere, biosphere, and geosphere and can be moved to another layer by biochemical processes. Within the soil, carbon can be compromised of many types of organisms and different chemical forms. There are many different laboratory procedures available to measure types of carbon in the soil. A certain procedure can be used if you are interested in a certain pool of carbon that is contained in the soil. A composite sample should be collected only if you want to send in one sample from a field, but grid sampling should be conducted to see if there is variation in soil carbon related properties across the field. There is interest in the soils ability to store carbon in the form of carbon stocks. Carbon credits are what the cost of a ton of CO2 is worth and what companies will pay agricultural producers to conduct practices that will increase soil carbon and remove CO2 from the atmosphere. Management practices that improve indicators of soil health that are related to carbon are retaining crop residues, reducing tillage, adding animal manure, and diversifying cropping systems. Future articles will be added to SDSU Extension's soil health library discussing soil carbon and the parts of the carbon cycle mentioned in this article.
References
- Bhattacharyya, S. S., Ros, G. H., Furtak, K., Iqbal, H. M., & Parra-Saldívar, R. (2022). Soil carbon sequestration–An interplay between soil microbial community and soil organic matter dynamics. Science of The Total Environment, 815, 152928.
- Bundy, L. G., & Bremner, J. M. (1972). A simple titrimetric method for determination of inorganic carbon in soils. Soil Science Society of America Journal, 36(2), 273-275.
- Cavigelli, M. A., Robertson, G. P., & Klug, M. J. (1995). Fatty acid methyl ester (FAME) profiles as measures of soil microbial community structure. In The Significance and Regulation of Soil Biodiversity: Proceedings of the International Symposium on Soil Biodiversity, held at Michigan State University, East Lansing, May 3–6, 1993 (pp. 99-113). Springer Netherlands.
- Cambardella, C. A., Gajda, A. M., Doran, J. W., Wienhold, B. J., Kettler, T. A., & Lal, R. (2001). Estimation of particulate and total organic matter by weight loss-on-ignition. Assessment methods for soil carbon, 349-359.
- Ćirić, V., Belić, M., Nešić, L., Šeremešić, S., Pejić, B., Bezdan, A., & Manojlović, M. (2016). The sensitivity of water extractable soil organic carbon fractions to land use in three soil types. Archives of Agronomy and Soil Science, 62(12), 1654-1664.
- Guo, Y., Wang, X., Li, X., Wang, J., Xu, M., & Li, D. (2016). Dynamics of soil organic and inorganic carbon in the cropland of upper Yellow River Delta, China. Scientific Reports, 6(1), 36105.
- Horváth, B., Opara-Nadi, O., & Beese, F. (2005). A simple method for measuring the carbonate content of soils. Soil Science Society of America Journal, 69(4), 1066-1068.
- Hurisso, T. T., Culman, S. W., Horwath, W. R., Wade, J., Cass, D., Beniston, J. W., ... & Ugarte, C. M. (2016). Comparison of permanganate‐oxidizable carbon and mineralizable carbon for assessment of organic matter stabilization and mineralization. Soil Science Society of America Journal, 80(5), 1352-1364.
- Joergensen, R. G., Wu, J., & Brookes, P. C. (2011). Measuring soil microbial biomass using an automated procedure. Soil Biology and Biochemistry, 43(5), 873-876.
- Kalbitz, K., Solinger, S., Park, J. H., Michalzik, B., & Matzner, E. (2000). Controls on the dynamics of dissolved organic matter in soils: a review. Soil Science, 165(4), 277-304.
- Kravchenko, A. N., Guber, A. K., Razavi, B. S., Koestel, J., Quigley, M. Y., Robertson, G. P., & Kuzyakov, Y. (2019). Microbial spatial footprint as a driver of soil carbon stabilization. Nature communications, 10(1), 3121.
- Lal, R. (2013). Soil carbon management and climate change. Carbon Management, 4(4), 439-462.
- Landgraf, D., Leinweber, P., & Makeschin, F. (2006). Cold and hot water–extractable organic matter as indicators of litter decomposition in forest soils. Journal of Plant Nutrition and Soil Science, 169(1), 76-82.
- Li, J., Wen, Y., Li, X., Li, Y., Yang, X., Lin, Z., ... & Zhao, B. (2018). Soil labile organic carbon fractions and soil organic carbon stocks as affected by long-term organic and mineral fertilization regimes in the North China Plain. Soil and Tillage Research, 175, 281-290.
- National Cooperative Soil Survey (2023). National Cooperative Soil Survey Soil Characterization Database. Accessed Thursday, November 9, 2023.
- Naylor, D., Sadler, N., Bhattacharjee, A., Graham, E. B., Anderton, C. R., McClure, R., ... & Jansson, J. K. (2020). Soil microbiomes under climate change and implications for carbon cycling. Annual Review of Environment and Resources, 45(1), 29-59.
- Nelson, D. W., & Sommers, L. E. (1996). Total carbon, organic carbon, and organic matter. Methods of soil analysis: Part 3 Chemical methods, 5, 961-1010.
- Paul, E. A., Morris, S. J., Conant, R. T., & Plante, A. F. (2006). Does the acid hydrolysis–incubation method measure meaningful soil organic carbon pools?. Soil Science Society of America Journal, 70(3), 1023-1035.
- Sellars, S., G. Schnitkey, C. Zulauf, K. Swanson and N. Paulson. "What Questions Should Farmers Ask about Selling Carbon Credits?" farmdoc daily (11):59, Department of Agricultural and Consumer Economics, University of Illinois at Urbana-Champaign, April 13, 2021
- Sharma, V., Hussain, S., Sharma, K. R., & Arya, V. M. (2014). Labile carbon pools and soil organic carbon stocks in the foothill Himalayas under different land use systems. Geoderma, 232, 81-87.
- Sherrod, L. A., Dunn, G., Peterson, G. A., & Kolberg, R. L. (2002). Inorganic carbon analysis by modified pressure‐calcimeter method. Soil Science Society of America Journal, 66(1), 299-305.
- Singh, M., Sarkar, B., Sarkar, S., Churchman, J., Bolan, N., Mandal, S., ... & Beerling, D. J. (2018). Stabilization of soil organic carbon as influenced by clay mineralogy. Advances in agronomy, 148, 33-84.
- Tobin, C., Singh, S., Kumar, S., Wang, T. and Sexton, P. (2020) Demonstrating Short-Term Impacts of Grazing and Cover Crops on Soil Health and Economic Benefits in an Integrated Crop-Livestock System in South Dakota. Open Journal of Soil Science, 10, 109-136.
- Vance, E. D., Brookes, P. C., & Jenkinson, D. S. (1987). An extraction method for measuring soil microbial biomass C. Soil biology and Biochemistry, 19(6), 703-707.
- Wade, J., Culman, S. W., Hurisso, T. T., Miller, R. O., Baker, L., & Horwath, W. R. (2018). Sources of variability that compromise mineralizable carbon as a soil health indicator. Soil Science Society of America Journal, 82(1), 243-252.
- Weil, R. R., Islam, K. R., Stine, M. A., Gruver, J. B., & Samson-Liebig, S. E. (2003). Estimating active carbon for soil quality assessment: A simplified method for laboratory and field use. American Journal of Alternative Agriculture, 18(1), 3-17.
- Witzgall, K., Vidal, A., Schubert, D. I., Höschen, C., Schweizer, S. A., Buegger, F., ... & Mueller, C. W. (2021). Particulate organic matter as a functional soil component for persistent soil organic carbon. Nature Communications, 12(1), 4115.
- Zhao, A., Zhang, M., & He, Z. (2013). Spectroscopic characteristics and biodegradability of cold and hot water–extractable soil organic matter under different land uses in subarctic Alaska. Communications in soil science and plant analysis, 44(20), 3030-3048.
- Zsolnay, A. (1996). Dissolved humus in soil waters. In Humic substances in terrestrial ecosystems (pp. 171-223). Elsevier Science BV.

