There are many different chemicals that make up plants. These chemicals include hydrogen, carbon, oxygen, nitrogen, phosphorus, sulfur, calcium, magnesium, iron, manganese, copper, boron, zinc, molybdenum, cobalt, and chlorine. The plants can get some of these elements from the atmosphere and water (carbon, hydrogen and oxygen), whereas others are most commonly taken up from the media plants are grown in. The nutrients that plants need the most are called macronutrients, which are nitrogen, phosphorous, potassium, calcium, magnesium, and sulfur. The micronutrients required by plants are iron, manganese, copper, boron, zinc, molybdenum, cobalt, and chlorine. The nutrients in soils that are the most limiting to plant growth are nitrogen, phosphorous, and potassium. Most of the time soil testing focuses on these three nutrients, with nitrogen usually being the most limiting of these nutrients.
Collection of Samples

The first step to finding how much of each element is contained in the soil is to collect a soil sample. Soils are usually sampled at the 0 to 6 inch depth in gardens. To conduct sampling, you will need the following items: A container to homogenize the sample in, a bag to place the homogenized sample in, and a device to collect the sample. Soils can be collected with a soil probe or a shovel, such as those shown in Figure 1.
Once you have the materials together to collect the soil sample, subsamples should be taken from spots throughout the garden. Figure 2 shows an example on how to collect a composite sample from a 1000 square-foot garden. A sample, either from a soil probe sample or a small shovel, is collected at locations 1 through 6 from the 0 to 6-inch depth. About the same volume of sample should be collected from each location.
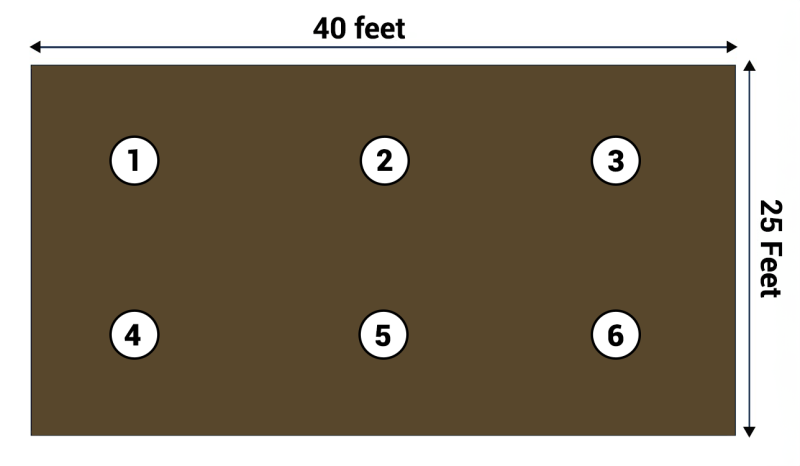
The sample from each of the locations in the garden is then placed into a container and is homogenized by mixing in the container. A subsample of the composite soil sample is then placed into the bag and sent off to the laboratory.
If you plan to grow crops that require a low pH, you should select a portion of the garden to devote to the low-pH crop. If sampling is done prior to committing a portion of the garden to growing crops that like low or high pH, collecting one composite sample is probably adequate.
However, if you have been using amendments that adjust soil pH, these portions of the garden should each be sampled separately. More details on sampling field soils and garden soils can be found on the publication page Recommended Soil Sampling Methods and Instructions by Clark (2019, 2020).
Calculating Nutrient Amount in Soil
When submitting samples to a soil testing lab, a routine or standard fertility analysis is usually adequate. A list of labs can be found in SDSU Extension’s Soil Testing Labs listing. A routine soil analysis will generally give you values for soil organic matter, nitrogen, phosphorous, potassium, pH, and electrical conductivity. Most gardens usually have adequate levels of micronutrients. If you suspect a micronutrient deficiency in soil, then a sample should be submitted to a laboratory to test for the specific micronutrient or a small amount of the micronutrient can be added to the soil.
An example of values from a soil test is shown in Table 1. The soil tests generally give you the concentration of the element in parts per million (abbreviated as ppm). Usually, field crop recommendations are given at a pounds (abbreviated as lbs.) per acre rate and garden recommendations are based on lbs. per 1000 ft2. The lbs. per acre application rate can be converted to lbs./1000 ft2 by dividing it by 43.56. To get from ppm to lbs. per acre in soil, you need an estimate or measurement of soil bulk density. The extension article, Bulk Density is an Indicator of Soil Health, by Klopp and Bly (2023) goes into more detail about bulk density. Multiply ppm of element × bulk density × soil depth × ft2 in an acre to get lbs. per acre. One can assume garden soil is about ½ pore space and ½ solids, which corresponds to a bulk density of about 1.3 g/cm3 or 81.3 lbs./ft3. Based off 6 ppm of nitrogen (nitrogen as ammonium + nitrogen as nitrate) listed in Table 1, multiplying 0.000006 parts N per part soil × 81.3 lbs. soil/ft3 x 0.5 ft. depth × 43560 ft2/ 1 acre determines that there are 10.6 lbs. of nitrogen per acre in the 0 to 6-inch depth.
|
|
|
|
|
|||
|
Soil Property |
Concentration |
Test Category |
Concentration |
Test Category |
Concentration |
Test Category |
| pH | 6.7 | ideal | 6.2 | low | 5.8 | Low |
| SMP Buffer pH | 7.2 | - | 6.6 | - | 6.8 | - |
| 1:1 Electrical Conductivity (dS/m) | 0.19 | low | 0.16 | low | 0.04 | Low |
| Organic Matter (%) | 4.8 | ideal | 5.5 | Ideal | 1 | low |
| Nitrogen as Nitrate (ppm) | 1.31 | low | 6.31 | low | 0.63 | low |
| Nitrogen as Ammonium (ppm) | 4.7 | low | 4.8 | low | 1.8 | low |
| Potassium (ppm) | 259 | very high | 228 | very high | 30 | very low |
| Phosphorus (ppm) | 36 | very high | 37 | very high | 82 | very high |
| Sulfate (ppm) | 10.4 | low | 11.8 | low | 10.6 | low |
| Zinc (ppm) | 0.55 | medium | 0.89 | high | 0.62 | medium |
| Iron (ppm) | 53.3 | high | 57 | high | 19.2 | high |
| Manganese (ppm) | 21.8 | high | 27.2 | high | 1.7 | high |
| Copper (ppm) | 0.83 | high | 0.81 | high | 0.12 | medium |
| Calcium (ppm) | 2836 | very high | 2249 | very high | 254 | medium |
| Magnesium (ppm) | 690 | very high | 564 | very high | 63 | very high |
| Cation Exchange Capacity (cmolc/kg soil) |
20.6 | - | 20.9 | - | 4.4 | - |
*Nutrients levels that are inadequate for garden production are in bold. NERF is Northeast Research Farm and CRP is Conservation Reserve Program Grassland.
Interpreting a Soil Test and Calculating Amount Needed
Different states or sources give you different ways to determine how much of a certain nutrient is needed. Some give you an equation to estimate how much fertilizer to apply based on estimated yield that can be obtained, which is called yield goal. Others give an estimate of lbs./ acre needed for growing the crop based on soil test intrepretation. Although different vegetable crops may need different quantities of nutrient levels for optimum yields, the SDSU extension fertilizer recommendations give one value to estimate fertilizer requirements (Warncke et al., 2004 and Clark 2023). There are equations available to estimate how much N, P and K needs to be applied to gardens based off the soil test level (Clark 2023). To determine lbs./ 1000 ft2 nitrogen that needs to be applied from a soil that contains 10.6 lbs. nitrogen/acre, you would place the value into the equation 3.5- (0.03 × Soil Test Nitrogen) which is (3.5- 0.03 × 10.6 lbs. nitrogen/acre). This determines that 3.18 lbs. of nitrogen fertilizer needs to be applied per 1000 square feet.
The fertilizer recommendation for phosphorous and potassium in the South Dakota Fertilizer Recommendations guide is based upon the soil test concentration in ppm. The equation to calculate the phosphorous requirement per 1000 square-feet uses the equation 3.6- (0.23 × soil test phosphorous), which for the NERFCRP sample is 3.6 - (0.23 × 36 ppm phosphorous). The value comes out to -4.68, which is a negative value and means that no phosphorous needs to be added. The equation to calculate the potassium needed is 5.4- (0.03 × soil test potassium in ppm). If you enter the potassium concentration on the same soil sample of 259 ppm, the equation calculates -2.37, which means no potassium needs to be added. The only one of the three samples that would need potassium in Table 1 is the sand sample. The sand sample, which has 30 ppm potassium (5.4 - 0.03 × 30 ppm potassium) would need 4.5 lbs. of K2O per 1000 square feet. Based on the SDSU Extension recommendation, the sand sample needs 3.37 lbs. of Nitrogen and 4.5 lbs. of K2O per 1000 square-feet. Other states, such as Wisconsin and Michigan, provide nutrient recommendations for specific vegetable crops (Warneke et al., 2004 and Laboski and Peters 2012). Some garden crops that can fix nitrogen, such as peas and beans, do not need nitrogen fertilization.
Fertilizers are sold as a percentage of the nutrient in the fertilizer solids. Nitrogen is calculated as percent nitrogen, phosphorus as P2O5 and potassium as K2O. If nitrogen and potassium are the only nutrients needed, fertilizers such as urea (CO(NH2)2) and potassium chloride (muriate of potash) would be used. Urea is 45-0-0 and Potash is 0-0- 60, which means the fraction of urea that is nitrogen is 0.45, and the fraction of potash that is potassium is 0.6. To calculate the amount of nutrient needed, divide the pounds of nutrient needed by fraction of the nutrient in the fertilizer. For nitrogen, it would be 3.37/0.45, and for potassium it would be 4.5/0.6, which would equate to adding 7.5 lbs. of Urea and 7.5 lbs of Potash per 1000 square-feet. If the soil pH value is between 6-7.5, it is ideal for most crops.
An extension publication that gives pH range for garden crops is in Tarkalson et al., (2005). The only soil in Table 1 that is below this range is the sand. However, when looking at lime recommendation in South Dakota, the buffer pH is above 6.5 (Clark 2023). This gives a lime recommendation of 0. Soils are considered saline if the electrical conductivity measured via saturated paste extract is greater than 4 dS/m. Most soil labs measure electrical conductivity via a 1:1 soil: water extract due to it being easier to measure. In the NRCS Soil Health Guide, it says if a 1:1 soil: water extract is < 1dS/m, there is not a salt issue (NRCS 2014). The three soils in Table 1 have electrical conductivity values much lower than this. The only other nutrient that is deficient in these samples is sulfate, which is about 18 to 20 lbs./acre in these samples collected from the 0 to 6-inch depth. The South Dakota Fertilizer Recommendation Guide suggests taking a deeper sample from a 6 to 24-inch depth prior to adding sulfur fertilizers. Sulfate is easily leached, and some of the deeper plant roots may uptake some of the sulfur from below the 6-inch depth.
References
- Clark (2023). Fertilizer Recommendations Guide. SDSU Extension. Revised from Original publication; Gerwig, J., Gelderman, R. (2005) Fertilizer Recommendations Guide. SDSU Extension.
- Clark (2020). SDSU Extension. Taking a lawn and carbon soil sample. SDSU Extension.
- Clark, J. (2019) Recommended Soil Sampling Methods for South Dakota. SDSU Extension. (Revised from Original publication: Gelderman, R., J. Gerwing, and K. Reitsma. (2006) Recommended Soil Sampling Methods for South Dakota. SDSU Extension.
- Klopp, H. W. & Bly, A. (2023) Bulk density is an indicator of soil health. SDSU Extension. SDSU Extension.
- Laboski, C. & Peters, J. 2012. Nutrient application guidelines for field, vegetable and fruit crops in Wisconsin. University of Wisconsin Cooperative Extension. Publication A2809
- NRCS (2014). USDA NRCS. Soil Health-Guides for Educators. USDA NRCS
- Tarkalson, D.D., Lindgren, D.T., Shapiro, C.A. & Hadges, L. 2005. Fertilizers for Vegetables in Home Gardens. University of Nebraska-Lincoln Extension. Publication G945.
- Warncke, D., Dahl, J., Zandstra, B. (2004) Nutrient Recommendations for Vegetable Crops in Michigan. Michigan State University Extension Extension Bulletin E2934.

