Nitrogen is an essential nutrient for plant growth and is generally the most-limiting plant nutrient in the soil. However, nitrogen gas makes up a large percent of the atmosphere. The atmosphere is about 78% nitrogen. Certain organisms (autotrophs) and plants (legumes) can take nitrogen that is contained in the atmosphere and convert it to organic nitrogen in the bacteria or plant. Some types of free-living bacteria (heterotrophs, such as Azotobacter and Clostridium) can take nitrogen from the air and directly convert it to organic nitrogen, but the amount of nitrogen converted is usually small, 0 to 25 pounds per acre, per growing season (Russelle, 2008). There also are bacteria that can create beneficial relationships with plant roots that benefit both the microorganism and the living plants. Examples of legume plants that create relationships with nitrogen-fixing bacteria include alfalfa, soybeans, other bean crops, peas, vetch, clovers, and sun hemp. If a nitrogen-fixing plant has not been planted at a location for a while, mixing some of the nitrogen-fixing bacteria with the seeds when planting can improve the plants’ performance. When planting crops that can fix large amounts of nitrogen, such as soybean (up to 400 lbs. N fixed per acre, per year) (Hungria & Mendes, 2015) no nitrogen fertilizer application is needed. These crops also can provide some nitrogen to the crop planted the following year, reducing the amount of nitrogen fertilizer needed. There also are non-symbiotic bacteria called diazotrophs that can live in plants or associate with roots in the soil. These can fix up to about 70 lbs. N per acre, per year and are an important nitrogen source in natural ecosystems (Gupta et al., 2019).
An example of the nitrogen cycle is in Figure 1. The process of converting nitrogen gas in the atmosphere to organic forms of nitrogen in plant and microbes is nitrogen fixation. During ammonification, organic forms of nitrogen are converted into ammonia (NH4+). Nitrification is the process which converts ammonia or ammonium (NH4+) into nitrate (NO3-). This process involves bacteria, which oxidize the reduced forms of nitrogen in ammonia (NH3) or NH4+ into a more-oxidized form of nitrogen as NO3-. This process most commonly involves Nitrosomonas bacteria species, which convert NH3 or NH4 to nitrite (NO2-), and Nitrobacter converts NO2- into NO3-. The nitrification process only occurs in environments where oxygen is present. In anaerobic soils or water, the denitrification process can occur. This process converts nitrate into nitrogen gas. Microbes use nitrogen as an electron source and convert nitrate to nitrogen gas. This process is carried out in four steps, which are included in the reactions below.
- H2 + NO3- + 2 e- → NO2- + H2O
- H2 + NO2- + 2 e- → NO + H2O
- H2 + NO + 2 e- → N2O + H2O
- H2 + N2O +2 e- → N2 + H2O
During the third step in this process, nitrous oxide (N2O) is produced, which is a gas. If this gas is able to move into the atmosphere, it adds to the concentration in the air. N2O is considered one of the greenhouse gases that people are concerned about. However, this reaction usually goes through the fourth step, which transforms N2O into nitrogen gas (N2). If the soil has a lot of NO3- in it and becomes anaerobic, the denitrification process can occur. This process may reduce the concentration of available nitrogen in the soil and may decrease the concentration of applied fertilizer in the soil, resulting in lower fertilizer nitrogen use efficiency and pollution of air and/or water.
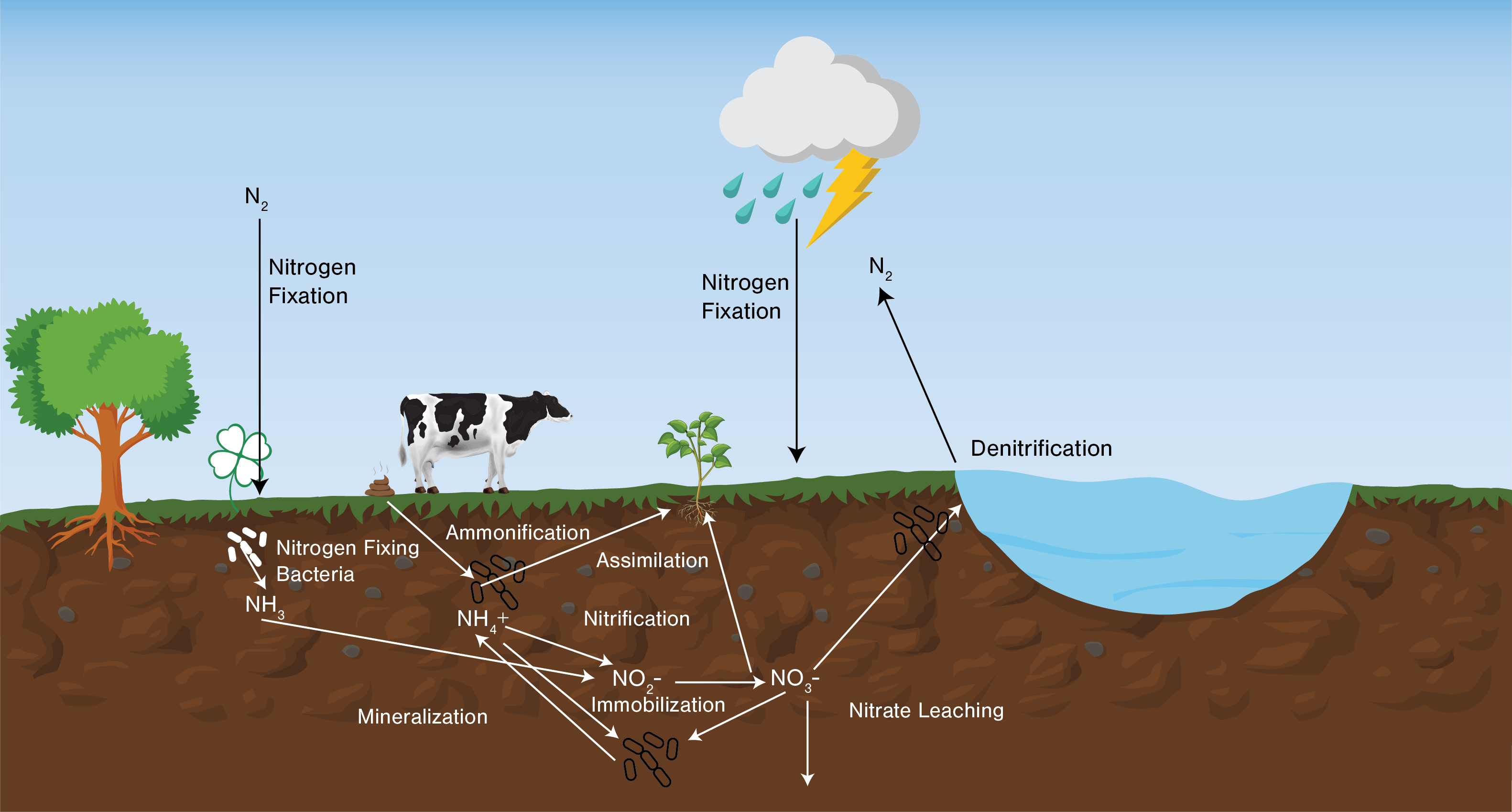
Soil biology breaks down carbon sources present in soil. Nitrogen is contained in organic carbon sources that are being broken down. The nitrogen that is contained in the organic carbon is broken down and can be converted into inorganic carbon and nitrogen (mineralization). Microbes are essential for the ammonification process to occur in the soil. Once ammonification occurs, the NH4+ can be taken up by plants, used by bacteria (immobilization), or transformed into nitrate. Bacteria have an ideal carbon: nitrogen ratio. Carbon sources present in the soil have differing carbon: nitrogen ratios. If there are high amounts of high carbon: nitrogen sources in the soil, microbes will use inorganic nitrogen present in the soil to break down the organic carbon in the soil. When this occurs, some of the inorganic nitrogen present in the soil will be transformed from available to organic forms (immobilized). However, as the microbial process continues in the soil, organisms could be broken down, and the nitrogen could be converted back into inorganic forms. Although soil organic matter can have varying levels of nitrogen, soils with higher organic matter content also have higher levels of total nitrogen. Soil organisms breaking down organic nitrogen in the soil and converting it to ammonium and nitrate create a lot of the nutrients that are taken up by plants, and sometimes this process provides much of the nitrogen required for a given crop during the growing season.
Some nitrogen types in soil are mobile, whereas others are immobile. Organic nitrogen compounds and NH4+ in the soil generally are only lost through wind and water erosion. NH4+ has a positive electron charge and is attracted to negatively charged clays and organic compounds, making it less mobile in the soil. Furthermore, NH4+ is subject to interlayer fixation by 2:1 clay minerals, such as illite and vermiculite (Nunes et al., 2019); in this case, some of this fixed NH4+ can be slowly released over time and be used by plants. However, NO3- is negatively charged and is not attracted to most clays and organic compounds in the soil and, therefore, is mobile in the soil and easily leached downward in the soil with water movement. This causes some of the producers’ applied nitrogen to be lost and leached out of the root zone. Also, too much nitrate in ground and surface waters can reduce their quality.
Although nitrogen occurs in both organic and inorganic forms, traditionally only soil NO3- and NH4+ concentrations in soils are measured. They are usually measured in a soil extract and converted into the amount of nitrogen in the soil (Maynard et al., 1993). However, soils have nitrogen contained in many types of forms. Traditionally the amount of NO3- and NH4+ measured in soil extract is used to determine how much nitrogen needs to be applied to the planted crop. Total nitrogen is the total amount of nitrogen in the soil and is usually measured with dry combustion methods (Wright and Bailey, 2001). Organic nitrogen can be determined from subtracting inorganic nitrogen (exchangeable NH4+ and NO3-, but also non-exchangeable NH4+) concentration from total nitrogen. Organic nitrogen in the soil is contained in many forms, including as particulate organic matter, microbes, soluble nitrogen compounds, such as amino and organic acids, and in humus. Nitrogen contained in dissolved organic forms can be determined by measuring water-extractable organic nitrogen on a total nitrogen analyzer for water (Ros et al., 2009). Nitrogen contained in microbes can be measured by fumigating the soil and seeing the change in nitrogen concentration before and after fumigation occurs (Hood-Nowotony 2010). Particulate organic matter nitrogen is measured by dispersing soil and separating sand-sized fraction from silt and clay-sized fraction (Yang et al., 2012). Then total nitrogen content of the sand sized fraction is determined by dry combustion. Another common measurement to analyze how the microbial community in the soil mineralizes organic nitrogen to inorganic nitrogen is potentially mineralizable nitrogen (Drinkwater et al.,1997). This is determined by measuring soil inorganic nitrogen concentration then placing the soil into a container wetted to a certain water content. Then the soil is left in the container for a duration of time while organic nitrogen is converted to inorganic nitrogen. Then, after a duration of time, the soil is removed from the container, and inorganic nitrogen is measured again. The concentration of nitrogen at the start of the incubation is subtracted from nitrogen concentration at the end of the incubation to determine the amount of nitrogen mineralized. This measurement is useful, because most of the nitrogen contained in the soil is in organic forms and can give an indication of how much will be converted to plant-available nitrogen during the growing season.
Nitrogen is added to the soil in both inorganic and organic forms. Organic fertilizers, such as compost or manures, contain nitrogen that is both inorganic and organic forms. The organic nitrogen that is in these fertilizers is slowly transformed from organic to plant-available forms of NO3 and NH4. Planting crops that can fix nitrogen also can provide nitrogen for the following crop. In conventional agriculture, nitrogen is most commonly applied in inorganic forms. Commonly applied nitrogen fertilizers include Anhydrous Ammonia (NH3), Urea (CO(NH2)2), Monoammonium Phosphate NH4 (H2PO4), Diammonium Phosphate ((NH4)2H2PO4), and UAN solutions (CO(NH2)2NH4NO3). Some of what is contained in these fertilizers are plant-available forms of nitrogen, whereas others are not in plant-available forms. Urea undergoes hydrolysis and is converted into ammonium. When NH3 is applied to the soil, it is usually converted to NH4 quickly. However, if applied on the surface, NH3 is a gas that can be lost from the soil. When fertilizers are applied, they are in inorganic forms. When they are in the soil, some of the nitrogen from the fertilizers are taken up by the plant, whereas other forms are used by microorganisms. Some of the nitrogen used by microorganisms is converted to organic nitrogen. This usually remains in the soil and can be converted back to inorganic nitrogen. However, nitrogen from applied fertilizers can be converted to a gas and lost if the denitrification process occurs or if NO3- is leached out of the soil profile.
Nitrogen is an essential element for plant growth and is contained in many forms in the soil. Soil microbiology is essential for transforming nitrogen into different forms in the soil. NO3- and NH4+ are the most-common forms of nitrogen that can be taken up by plants. Some plants can fix nitrogen from the atmosphere, whereas others uptake inorganic nitrogen from the soil. Microbes can convert organic forms of nitrogen into inorganic forms, which can be taken up by plants. Inorganic nitrogen can be lost from the soil by NO3 leaching and denitrification process. Nitrogen can be added to the soil in both organic and inorganic forms.
References
- Drinkwater, L. E., Cambardella, C. A., Reeder, J. D., & Rice, C. W. (1997). Potentially mineralizable nitrogen as an indicator of biologically active soil nitrogen. Methods for assessing soil quality, 49, 217-229.
- Gupta, V. V., Zhang, B., Penton, C. R., Yu, J., & Tiedje, J. M. (2019). Diazotroph diversity and nitrogen fixation in summer active perennial grasses in a Mediterranean region agricultural soil. Frontiers in molecular biosciences, 6, 115.
- Hood-Nowotny, R., Umana, N. H. N., Inselbacher, E., Oswald-Lachouani, P., & Wanek, W. (2010). Alternative methods for measuring inorganic, organic, and total dissolved nitrogen in soil. Soil Science Society of America Journal, 74(3), 1018-1027.
- Hungria, M. and Mendes, I.C. (2015). Nitrogen Fixation with Soybean: The Perfect Symbiosis?. In Biological Nitrogen Fixation, F.J. de Bruijn (Ed.).
- Maynard, D. G., Kalra, Y. P., & Crumbaugh, J. A. (1993). Nitrate and exchangeable ammonium nitrogen. Soil sampling and methods of analysis, 1, 25-38.
- Nunes, V.L.N., Mulvaney, R.L. and Griesheim, K.L. (2019), Simple Diffusion Methods for Determination of Nonexchangeable Ammonium in Soils. Soil Science Society of America Journal, 83,1421-1430. https://doi.org/10.2136/sssaj2019.05.0167
- Ros, G. H., Hoffland, E., Van Kessel, C., & Temminghoff, E. J. (2009). Extractable and dissolved soil organic nitrogen–A quantitative assessment. Soil Biology and Biochemistry, 41(6), 1029-1039.
- Russelle, M.P. (2008). Biological Dinitrogen Fixation in Agriculture. In Nitrogen in Agricultural Systems (eds J.S. Schepers and W.R. Raun).
- Wright, A. F., & Bailey, J. S. (2001). Organic carbon, total carbon, and total nitrogen determinations in soils of variable calcium carbonate contents using a Leco CN-2000 dry combustion analyzer. Communications in Soil Science and Plant Analysis, 32(19-20), 3243-3258.
- Yang, X. M., Xie, H. T., Drury, C. F., Reynolds, W. D., Yang, J. Y., & Zhang, X. D. (2012). Determination of organic carbon and nitrogen in particulate organic matter and particle size fractions of Brookston clay loam soil using infrared spectroscopy. European Journal of Soil Science, 63(2), 177-188.


