Have you ever wondered whether a homemade salad dressing is safe when you’re eating it at your local picnic, potluck dinner, or at a family get together? How can you know whether or not the homemade recipe and processing of the salad dressing is safe? In this article, we will explore what food safety characteristics need to be addressed to ensure that a salad dressing is made safely.
What Is a Salad Dressing?
The FDA describes a salad dressing as an emulsified semisolid food prepared from vegetable oil(s), an acidifying agent such as vinegar, an egg yolk containing ingredient, and other optional ingredients such as salt, spices, stabilizers and thickeners and more. Standardized and non-standardized food dressings and condiment sauces are excluded from the FDA regulation 21 CFR Part 114 Acidified Foods (1). Although the FDA has given direction on the definition of a salad dressing, there is little guidance from the FDA as to the definition of a standardized and non-standardized food dressing. Additionally, the compositional characteristics and conditions for processing and packaging these products to produce a safe and shelf stable product are not clearly specified. Products in this category are typically acidic with a pH ≤4.6. Having a pH ≤4.6 will help inhibit the germination of spore forming pathogens, but most importantly, clostridium botulinum. The characteristics of a salad dressing such as pH, ingredients that are bacteriostatic/bactericidal, or a thermal treatment are all important in determining the safety of the food.
Dressing and sauces will typically contain vinegar. The vinegar contains varying amounts of acetic acid. Acetic acid plays an important role in the product’s characteristics as well as playing a food safety role. Acetic acid or other weak organic acid containing products usually rely on the weak organic acid content as part of the food safety and commercial sterility of the product. Weak organic acids, and in particular, acetic acid, have been shown to create conditions where bacteriostatic and bactericidal effects occur for vegetative pathogens. The acetic acid has a significant quantity of the acid that does not dissociate. It is the undissociated acid that is often shown to be one of the main contributors to the bacteriostatic and bactericidal effects of the acid.
Pathogens of Concern
Since dressings have a pH lower than 4.6, spore-forming pathogens are not of a concern. Additionally, a large majority of these products are formulated with vinegar which has acetic acid. As mentioned above, weak organic acids have been shown to create conditions where bacteriostatic and bactericidal effects occur for vegetative pathogens. Staph aureus would not be considered since the main concern is enterotoxin formation that requires growth to high levels, while with acetic acid acidification, will not grow below a pH of 4.5 (1). Enterohemorrhagic Escherichica coli (EHEC) has also been sometimes dismissed as a likely pathogen of concern due to the ingredients usually associated with dressing and sauces (1). The current industry practice is to use pasteurized eggs, therefore removing that EHEC risk. Listeria, Salmonella spp., and EHEC should all be considered as organisms of concern for dressings and condiment sauces products. Some spoilage organisms could potentially raise the pH of the product above 4.6 and thus allow for the potential of spore forming pathogens to grow out. Therefore, risk assessments need to be comprehensive. They need to take into considerations recent outbreaks, literature studies, and GMP’s along with bacteriostatic or bactericidal conditions from either the formula or the processing conditions.
Generally E. coli and Listeria are the more resistant pathogens, with Listeria being the most resistant (2, 3). However, it is the presence of acetic acid, low pH, salt, and natural antimicrobials that create a harsh environment for foodborne bacterial pathogens such as Salmonella, E. coli O157:H7, L. monocytogenes, and Staphylococcus. Products that use different acids may have a different pH and different amount of undissociated acid. Lastly, it is important to note that although the food safety characteristics of salad dressings creates a harsh environment for growth, it is never a bad idea to build additional food safety parameters in place such as adding a heat treatment step which can ensure the destruction of pathogens as well as lengthen the shelf life by destroying spoilage organisms.
Literature Values
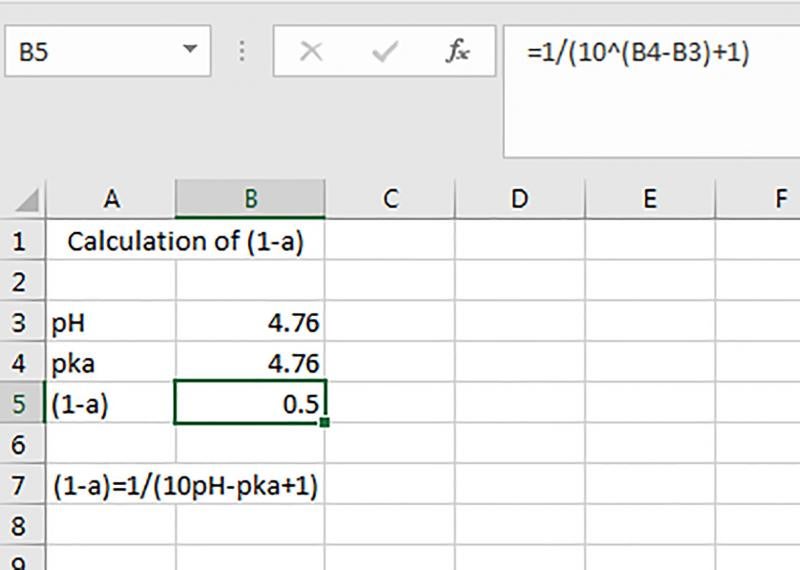
Literature studies have shown that products can generally be considered as bacteriostatic if they have a pH ≤4.5 and undissociated acetic acid >0.2% in the aqueous phase (2, 4). Products can generally be considered bactericidal if the pH ≤4.1 and acetic acid >0.7% in the aqueous phase (5). Lastly, products with a pH ≤4.4 and acetic acid >2.0% in the aqueous phase (6). The pH of a product can be tested using a pH meter. To calculate the amount of undissociated acetic acid you can put the following formula into excel as illustrated in Figure 1 (this formula assumes the use of vinegar as the acidulant). Targeting a formula that is bactericidal is the best option to ensure that a salad dressing is safe.
Heat Treatment
It is a good idea to ensure that the salad dressing receive a heat treatment especially if the product is bacteriostatic and even if it is bactericidal. This will help ensure that spoilage organisms are killed which will help lengthen the shelf life of the product and ensure that the product is safe. Listeria monocytogenes is used as the target pathogen since in most studies, it is the more heat resistant pathogen. The table below gives information on time and temperature conditions needed to achieve a 6 log kill or taking 1,000,000 cells of Listeria down to 1 cell. Not all products will rely solely on a thermal treatment or a bactericidal formula to achieve a food safe product. However, when a bactericidal formula and a heat treatment are used in combination, this will provide additional hurdles to ensure a safe product.
|
Temperature (F°) |
Temperature (C°) |
Rate |
Process (Minutes) |
|---|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Particulate Addition
If particulates are added, then it should be noted that before taking a pH of the product, the particulates need to reach equilibrium with the rest of the formula which can take 24 hours of being exposed to the acidic environment. This is especially important if the product contains a large amount of particulates or large particulates.
Regulatory Differences
If salad dressings are to be sold at the Farmer’s Market in South Dakota or to a retail establishment, they need to be processed out of a licensed commercial kitchen and evaluated by the South Dakota Department of Health. South Dakota State University Extension can test the pH of the product for $10 and can help evaluate the safety of the product as well to ensure that the product is safely formulated and processed.
Terms
- Acid Foods: Food that have a natural pH of 4.6 or below.
- Bacteriostatic: Means a set of conditions under which microorganisms will not grow or reproduce.
- Bactericidal: Means a set of conditions under which microorganisms will die off.
- Undissociated acid: Is the amount of acid in an aqueous solution that remains in its native form and does not dissociate into its hydrogen ion and conjugate base components. Weak organic acids, such as acetic acid can have large percentages of the acid that do not dissociate at a given pH whereas strong acids such as hydrochloric acid will have little content that does not dissociate.
Document Sources:
- Smittle, R.B. 2000. Microbiological safety and mayonnaise, salad dressings, and sauces produced in the United States: a review. J. Food Prot. 63:1144-1153.
- Beuchat, L.R., Ryu, J., Adler, B.B., and Harrison, M.D., 2006, Death of Salmonella, Escherichia coli O157:H7, and Listeria monocytogenes in Shelf-Stable Dairy-Based, Pourable Salad Dressings. J. Food Prot., Vol. 69, No. 4, Pages 801-814.
- Rhee, M., Less, S., Dougherty, M., and Kang, D. 200 Antimicrobial Effects of Mustard Flour and Acetic Acid against Escherichia coli O157:H7, Listeria monocytogenes, and Salmonella enterica Serovar Typhimurium. Appled and Environmental Microbiology, May 2003, p. 2959-2963.
- Mullan, M.W.A. (2009). Predicting the safety of products preserved using acetic acid.
- Glass, K.A., and M.P. Doyle. 1991. Fate of Salmonella and Listeria monocytogenes in commercial reduced-calorie mayonnaise. J. Food Prot. 54:691-69
- Association for Dressings and Sauces. 2014. Quality Assurance Guidelines – Section IV Microbiological Guidelines.
- Fish and Fishery Products Hazards and Controls Guidance 4th Edition. (2018 August).


