Written by Addie Stamps, former SDSU Extension Livestock Production and Stewardship Field Specialist.
If you have attended an in-person Beef Quality Assurance (BQA) training in South Dakota, you have heard about injection site lesions in beef cattle. We often talk about this as one of the first success stories of the BQA program. Since the initiation of the National Beef Quality Audit (NBQA), the percentage of animals with injection site lesions present in processing facilities has been monitored. The 2022 NBQA found that 97.1% of cattle processed in the market cow and bull facilities surveyed had no visible injection site lesions. Even better, 99.8% of cattle processed in the fed cattle processing plants surveyed had no visible injection site lesions. While those are great percentages, we never want to stop learning and improving.
What Causes Injection Site Lesions?

Injection site lesions form in the muscle where medication has been given to cattle. Typically, the worse reactions occur when an injection is given intramuscular or non-tented subcutaneously. These lesions can look vastly different in appearance depending on the injection given and the animals’ stage of life.
Metallic and nodular lesions frequently occur in cattle mid-to-late in the feeding phase when pharmaceuticals are given. Cystic lesions, however, occur when any injection is given, but typically in the finishing phase. Clear lesions and woody calluses commonly occur in young animals early in their lives regardless of what product is administered. These lesions are like the hide of the calf, they will continue to grow with the animal. As with any animal health concern, the severity of the lesions depends on each individual animal’s age, response to the product, contamination or adjuvant in the vaccine.
Injection Management Guidelines
As lesions can cause damage to costly beef cuts, the BQA program aims to educate producers on proper injection site management.
Injection Site
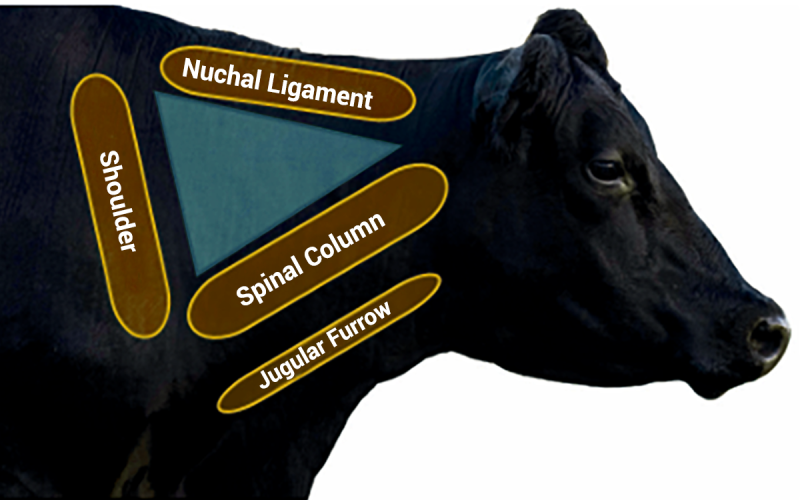
Injections should be given in the BQA injection site triangle (Figure 1), unless directed otherwise by a veterinarian or per label instruction. Adequate working facilities make this much easier, as restraining animals properly provides good neck access.
Per BQA guidelines, subcutaneously (SQ) injections should not be given along the ribs, behind the elbow, or in the armpit region of beef cattle. There are many nerves and vessels in these regions that could be damaged, causing irreversible injury to the animal. Above the curve of the ribs is not an approved injection site.
Injection Spacing
Injections should be spaced out at least 4 inches apart. The injection with the highest volume should be given at the lowest point, so that if the product drains it does not mix with other products.
Vaccines and antibiotics should be administered on opposite sides of the neck so that vaccines are not inactivated.
Route of Administration
Animal health products injected subcutaneously (SQ) are least likely to cause adverse reactions. However, always follow label directions regarding route of administration, whether that be SQ, intramuscular (IM), intravenous (IV), intranasal (IN), intramammary (IMM), base of ear (BOE), topical, or orally (see Table 1).
| Route | Location |
|---|---|
| Subcutaneously (SQ) | Under the skin, 35 to 45 degree angle. |
| Intramuscular (IM) | In the muscle, perpendicular to muscle, 90 degrees to neck. |
| Intravenous (IV) | In the vein. |
| Intramammary (IMM) | In the mammary gland, no needle. |
| Base of Ear (BOE) | Under the skin. |
| Intranasal (IN) | In the nose, no needle. |
| Topical | On the skin, transdermal. |
| Orally | Into the mouth. |
By following these guidelines, hopefully injection site lesion numbers found in beef cattle will remain low and good quality meat will continue to be produced.


